41 so2 pi bonds
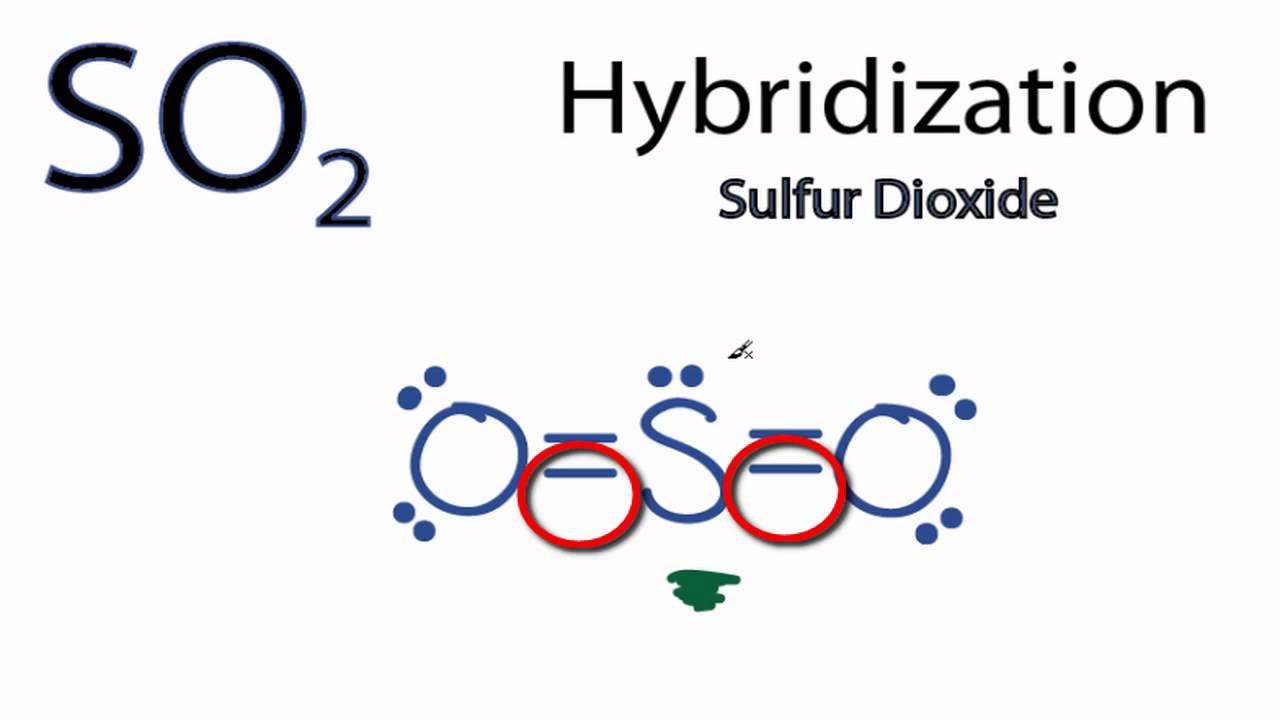
How many pi bonds are in SO_2? | Socratic There are two pi bonds in a single "SO"_2 molecule. First, let us consider the structure of the "SO"_2 molecule: As you can see, the molecule is bent / v-shaped / angular, and there are three regions of electron density: two "O = S" double bonds and a lone pair of electrons. Now, recall that the composition of a double bond is as follows: 1x sigma bond 1x pi bond Therefore, with two double ... How many pi bonds are there in "CO"_2? | Socratic Jan 3, 2015 CO2 has 2 pi bonds. First, start with the molecule's Lewis structure, which allows you to determine the hybridization of each atom. We can see that C has two regions of electron density around it, which means it has a steric number equal to 2.
How many pi bond in so2? - Answers How many pi bonds does SO2 have? There are two double bonds.So there are two pi bonds. Is SO2 a polar bond? SO2 contains polar sulfur-oxygen bonds, but no compound, such as SO2, is itself a bond at...

So2 pi bonds
How to find number of d-pi p-pi bondings in a compound. Is ... - Quora Lone pairs= (1/2)× (valence electrons - bonding electrons) σ bonds= no. of surrounding atoms π bonds= (no. of oxygen atoms) - (no. of negative charge) Hybridization = σ bonds + no. of lone pairs Now, no. of pπ-pπ bonds= no. of unhybridised p orbitals left pπ-dπ bonds will be formed when π bonds are more than the no. of unhybridised p orbitals left. Which of the following molecules is having 2ppi - 3dpi bond? P pi p pi bond in a SO2. Open in App. Solution. Verified by Toppr. Solve any question of The p-Block Elements with:-Patterns of problems > Was this answer helpful? 0. 0. Similar questions. Number of P pi - D pi bonds in So2 and SO3 - YouTube #NEETCHEMISTRY #IITJEECHEMISTRY Number of P pi - D pi bonds in So2 and SO3 .....Clear explanation in a simple manner which is useful for Competitive exami...
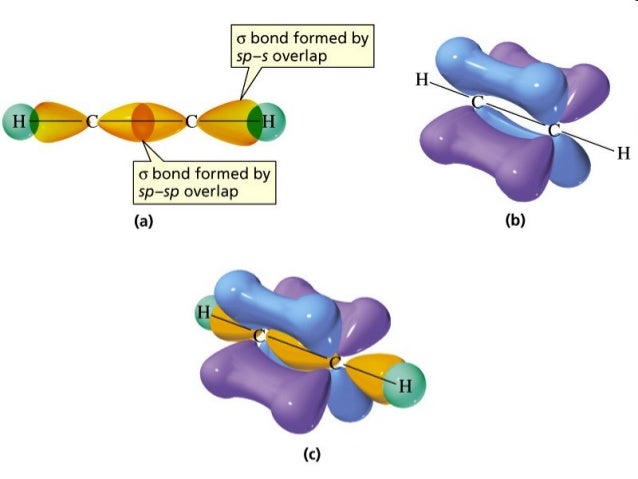
So2 pi bonds. SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram The molecular geometry of SO2 is bent, with a bond angle of 120°. We can easily find out the molecular geometry of any compound using the given chart. Here, A = central atom, X = surrounding atoms and E = the lone pairs. SO2 is an AX2E type molecule, with 2 surrounding atoms i.e oxygen, and 1 lone pair of sulfur. Is SO2 Ionic or Covalent? - Techiescientist The SO2 comprises 1s and 2p orbitals hybridized. Owing to the double Covalent bond of the molecule Sulphur Dioxide, it has 2 sigma and 2 pi bonds. SO2 has a bent structure and is not linear in nature. The bond angle being 120° SO2 becomes a polar molecule as the charges are unable to cancel the effects of each other. Pi Bond - Definition, Explanation, Examples with Illustrations The sp 2 hybridized carbons now form three sigma bonds and one pi bond, as illustrated below. The sp 2 hybridized orbital in the carbon atom is made up of a 2s electron, a 2p x electron, and a 2p y electron. It can form a total of three sigma bonds. The 2p z electrons of the carbon atoms now form a pi bond with each other. How do we find the bond order of SO2 (Sulphur Dioxide)? Hence in this case, one can find bond order by using the formula 1 + (Pi/Sigma), here sigma denotes the number of sigma bonds and pi denotes the number of pi bonds. In this case pi bonds are 1 and sigma bonds are 2, substituting it in the formula we can arrive at the answer, 1.5. Was this answer helpful? 2 (3) (3) (1)
SO2 Lewis Structure, Molecular Geometry, Hybridization, Polar or ... There is a total of three sp2 hybrid orbitals where two hybrid orbitals contain unpaired electrons, and one hybrid orbital has the lone pair of electrons. The unpaired electrons on sulfur form a sigma bond with oxygen atoms. Here both 3d and 3p orbitals remain the same and form Pi bonds within the molecule. SO2 MO Diagram . Summary inorganic chemistry - Why are the two bonds in sulphur dioxide ... The sulfur-oxygen bond has a bond order of 1.5 and does not invoke d orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. ( Wikipedia) However, there is a molecular orbital approach which seems to give a deeper appreciation of the bonding. The picture is this: SO2 Polar or Nonpolar - What's Insight Sulfur dioxide (SO2) is polar in nature. The electronegativity difference between sulfur (2.58) and oxygen (3.44) atoms makes it a polar molecule. ... The SO2 molecular geometry is bent, with a bond angle of 120°. ... one sigma and one pi bond are formed. To reduce repulsions, the double bonds and the lone pair are separated as much as ... sp² hybridized orbitals and pi bonds (video) | Khan Academy For a carbon with 1 double bond and 2 single bonds, the orbitals will become 33% "s" and 66.7% "p" making it "sp2." If there is a triple bond and a single bond, the orbitals will adjust again to become 50% "s" and 50% "p." So to summarize - You can find sp3 bonding when a carbon has 4 single bonds.
SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry ... There are two covalent bonds and one lone pair. There are three electron domains, and this gives SO 2 an sp 2 hybridization. Therefore, the hybridization of Sulfur Dioxide is sp 2. SO2 Bond angles. According to the VSEPR theory, the Oxygen atoms are repelled by each other and the lone pair, thus forming a bent molecular shape. no of p pi p pi and p pi d pi bonds in so2 - askIITians There are 3 (Pπ-Pπ ) & 1 (Pπ-dπ) Bonds In SO2 Molecule. Because 3 orbitals of P-subshell of S Overlap With 3 Orbitals of P-subshell of O2 & 1 Orbital of d-subshell of S Overlap With 1 orbital of P-subshell of O2........ Hence Proved......🙌🏻 ️ Provide a better Answer & Earn Cool Goodies See our forum point policy How many pi bonds are in SO2 class 11 chemistry CBSE Sigma and pi bonds are among the types of covalent bonds that can be distinguished by the type of overlap between 2 atomic orbitals. Covalent bond is generally formed by overlapping of orbitals (or we may say by sharing electron pairs). -Sigma Bond ( σ ): These are the strongest type of covalent bond because they are formed by h... Read More The number of dpi - ppi bonds present respectively in SO2, SO3, ClO4^- are: The number of dπ−pπ bonds present respectively in SO 2,SO 3,ClO 4− are: A 0, 1, 2 B 1, 2, 3 C 2, 3, 4 D 2, 3, 3 Medium Solution Verified by Toppr Correct option is B) The number of dπ−pπ bonds present in SO 2,SO 3,ClO 4− are 1,2,3 respectively. Video Explanation Was this answer helpful? 0 0 Similar questions σ π
What is the total number of pπ-pπ bonds in SO4(2-)? - Quora There are 0 P pi- P pi bonds present in ( SO4)2- BCZ in its structure two oxygen atoms are attached to sulphur with double bond and two oxygen having -1 charge on them are attached with single bond with sulphur atom. So the ion has 4 sigma bonds and 2 P pi- d pi bonds present. Hope you got it. Thank you. 13.2K views View upvotes Akansh Maurya
How many pi bonds in no2? - Answers How many pi bonds does SO2 have? There are two double bonds.So there are two pi bonds. How many sigma and pi bonds in methane? there are 4 sigma bonds and no pi bond.
Why p-π and d-π bonds in so3 sulphur is in excited state? Why p-π and d-π bonds in so3 sulphur is in excited state? - 5009392 aasthakansal353 aasthakansal353 04.08.2018 Chemistry ... electron to form bonds now 3 of the bonds are clearly sigma bonds made by one s and 2 p orbitals so there will be 1 p pi and 2 d pi bonds . Advertisement
Is SO2 have ppi -dpi bond | EduRev NEET Question In SO2 there are one p-pi and one p-pi-dpi bond Upvote | 1. Reply; Share Report Share. Answer this doubt. yes.... In SO2 there are one p-pi and one p-pi-dpi bond Top Courses for NEET. Biology Class 11; Biology 31 Years NEET Chapterwise Solved Papers; NEET Mock Test Series; NCERTs for NEET; Biology Class 12 ...
The sum of pi bonds in So2,So3 and (So3)3 (cyclic trimer) is सरल कीजिए3 (iPIN+coI . 1. On the evening of an autumnal equinox day Siddhant noticed that Mars wasexactly along the north-south meridian in his sky at the exact moment when …. the sun wassetting. In other words, the Sun and Mars subtended an angle of exactly 90 as measuredfrom the Earth. If the orbital radius of Mars is 1.52 au.
SO2 Ionic or Covalent?| Simple Explanation - What's Insight SO2 has a bent molecular shape, with a bond angle of 120°. The distribution of charges on the central atom is asymmetrical. ... Between sulfur and each oxygen atom, one sigma and one pi bond are formed between sulfur and each oxygen atom. To reduce repulsions, the double bonds and the lone pair are separated as much as possible, and so the ...
SO2 bond sigma and pi bond - CHEMISTRY COMMUNITY The Lewis Structure for SO 2 is a central Sulfur double bonded to each of the Oxygen atoms. A double bond consists of one sigma bond and one pi bond so in total you would have two sigma bonds and two pi bonds (one sigma and pi for one bonded Oxygen and another sigma and pi for the other). Top Sebastian Lee 1L Posts: 157
inorganic chemistry - How does SO2 have 2 π bonds? - Chemistry Stack ... The hybridization of sulfur atom is sp2 hence a lone pair and two bond pairs(due to sigma bonding) reside in these hybrid orbitals. The unpaired electrons are 3p and 3d hybridized orbitals are used in pi bonding with oxygen's unhybridized 2p orbitals. Hence two pi bonds are formed which are p-p pi and d-p pi bonds.
SO2 Hybridization - YouTube A description of the hybridization of SO2 including sigma and pi bonds.Note that the SO2 hybridization is sp2 for the central sulfur atom. It's also sp2 fo...
Post a Comment for "41 so2 pi bonds"